Graphene Jolts Sodium-Ion Batteries’ Capacity
Right after many years of anticipation, sodium-ion batteries are starting to produce on their assure for energy storage. But so significantly, their commercialization is restricted to significant-scale makes use of these kinds of as storing energy on the grid. Sodium-ion batteries just don’t have the oomph essential for EVs and laptops. At about 285 Wh/kg, lithium-ion batteries have 2 times the energy density of sodium, producing them far more appropriate for people portable applications.
Researchers now report a new style of graphene electrode that could raise the storage ability of sodium batteries to rival lithium’s. The materials can pack just about as quite a few sodium ions by volume as a standard graphite electrode does lithium. It opens up a route to producing low-charge, compact sodium batteries functional.
Considerable and low cost, and with similar chemical attributes as lithium, sodium is a promising substitution for lithium in future-era batteries. The stability and security of sodium batteries helps make them particularly promising for electronics and vehicles, where by overheated lithium-ion batteries have in some cases demonstrated dangerous.
“But currently the main problem with sodium-ion batteries is that we don’t have a appropriate anode materials,” states Jinhua Sunshine, a researcher in the division of industrial and elements science at Chalmers University of Technological innovation.
For the battery to demand quickly and retailer a good deal of energy, ions need to have to simply slip in and out of the anode materials. Sodium-ion batteries use cathodes made of sodium steel oxides, when their anodes are commonly carbon-centered anodes just like their lithium cousins although Santa Clara, California-centered Natron Energy is producing both of those its anodes and cathodes out of Prussian Blue pigment utilised in dyes and paints.
Some sodium battery builders are utilizing activated carbon for the anode, which retains sodium ions in its pores. “But you need to have to use high-grade activated carbon, which is very pricey and not simple to make,” Sunshine states.
Graphite, which is the anode materials in lithium-ion batteries, is a reduce charge choice. Even so, sodium ions do not move effectively amongst the stack of graphene sheets that make up graphite. Researchers utilised to consider this was for the reason that sodium ions are larger than lithium ions, but turns out even-larger potassium ions can move in and out simply in graphite, Sunshine states. “Now we consider it is really the surface chemistry of graphene layers and the electronic framework that simply cannot accommodate sodium ions.”
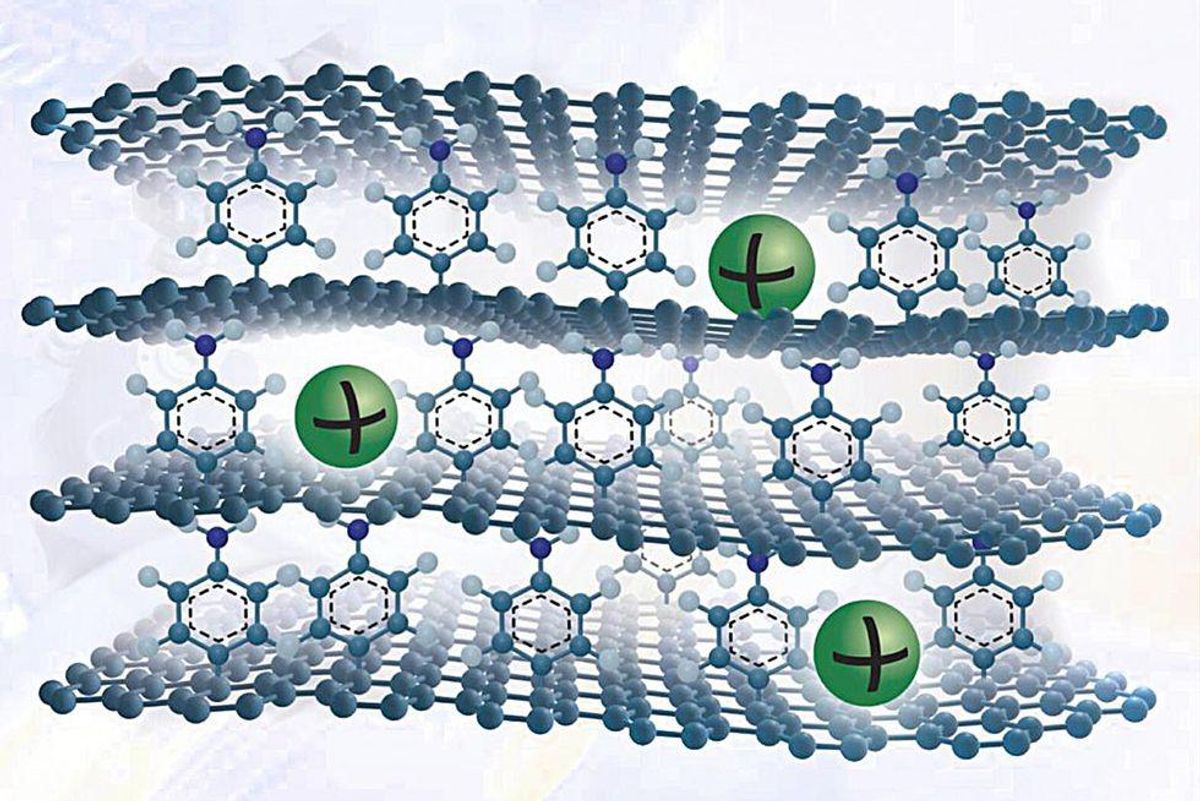
He and his colleagues have come up with a new graphite-like materials that overcomes these difficulties. To make it, they develop a single sheet of graphene on copper foil and connect a single layer of benzene molecules to its major surface. They develop quite a few these kinds of graphene sheets and stack them to make a layer cake of graphene held apart by benzene molecules.
The benzene layer raises the spacing amongst the layers to allow for sodium ions to enter and exit simply. They also build defects on the graphene surface that as as active response sites to adsorb the ions. Additionally, benzene has chemical groups that bind strongly with sodium ions.
This seemingly simple method boosts the material’s sodium ion-storing ability considerably. The researchers’ calculations show that the ability matches that of graphite’s ability for lithium. Graphite’s ability for sodium ions is commonly about 35 milliAmpere-hrs for every gram, but the new materials can hold above 330 mAh/g, about the same as graphite’s lithium-storing ability.